Molecular symmetry
Research on molecular symmetry focusses on the application of the molecular symmetry group whose elements consist of nuclear permutations with and without the inversion.The second edition of the book `Molecular Symmetry and Spectroscopy' has been written in collaboration with P. R. Bunker, and this was published by NRC RESEARCH PRESS in August 1998. The first edition, written by P. R. Bunker, was published by Academic Press in 1979.
`Molecular Symmetry and Spectroscopy' is now available either as e-book from NRC RESEARCH PRESS or as print-on-demand hardcopy from the company Volumes (in Kitchener, Ontario, Canada).
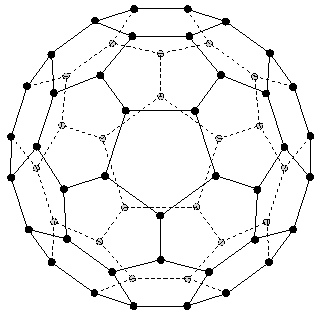
The molecular symmetry group of the molecule C<sub>60</sub> is the icosahedral group <b>I</b><sub>h</sub>(M) with 120 elements.
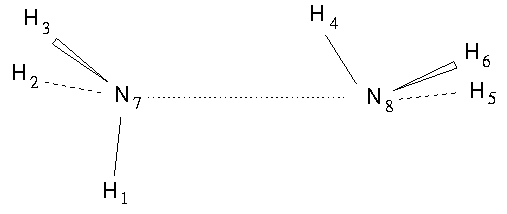
The ammonia dimer (NH<sub>3</sub>)<sub>2</sub> , however, has the molecular symmetry group <b>G</b><sub>144</sub> with 144 elements. Thus we can argue that (NH<sub>3</sub>)<sub>2</sub> has higher symmetry than C<sub>60</sub>. The symmetries of C<sub>60</sub>, (NH<sub>3</sub>)<sub>2</sub> and of many other molecules are discussed in `Molecular Symmetry and Spectroscopy'.

A Russian translation of "Molecular Symmetry and Spectroscopy" by Drs. Yurii N. Panchenko, Sergei V. Petrov, Vladimir I. Pupyshev, Andrei V. Scherbinin, and Prof. Nikolai F. Stepanov (editor) has been published by MIR, Moscow, in May 2004.
Click here for ordering information (in Russian).
An Indian edition was published in 2005. Contact overseas[at]del3.vsnl.net.in for information.
Fundamentals of Molecular Symmetry

Fundamentals of Molecular Symmetry by Philip R. Bunker and Per Jensen was published by IOP Publishing, Bristol, UK, in November 2004 (ISBN 0-7503-0941-5).
The book has 358 pages and costs US$ 60.95.
The book-publishing part of IOP Publishing has been taken over by Taylor and Francis. Click here for the Taylor and Francis presentation of the book.
Click here to see the amazon.com presentation of the book.
In Chemistry World vol. 3 (March 2005), "Fundamentals of Molecular Symmetry" is reviewed by Mark Child:
". . . . In summary, this book is welcome, first as an illuminating graduate text and also as an addition to the shelves of anyone who is responsible for teaching molecular symmetry at whatever level."
In CHOICE Reviews (July 2005), Mark Marshall says:
".... Summing Up: Highly recommended.
D. Smith writes in Contemporary Physics (September 2005) that:
"Fundamentals of Molecular Symmetry is an excellent book. ... [It] should be an essential text for any graduate student or established researcher working in the area of high-resolution spectroscopy. ...Fundamentals of Molecular Symmetry is exceptionally well written and up to the very high standards set by the authors in their previous texts. ...[The] figures and diagrams are beautifully produced. ... Readers will also particularly appreciate the way each concept is illustrated by examples of application to real molecular systems. ...In short, Fundamentals of Molecular Symmetry has my unqualified recommendation."
A new initiative is the Internet lecture course "Fundamentals of Molecular Symmetry - The lecture course". Click here for a description.
The molecular superrotor
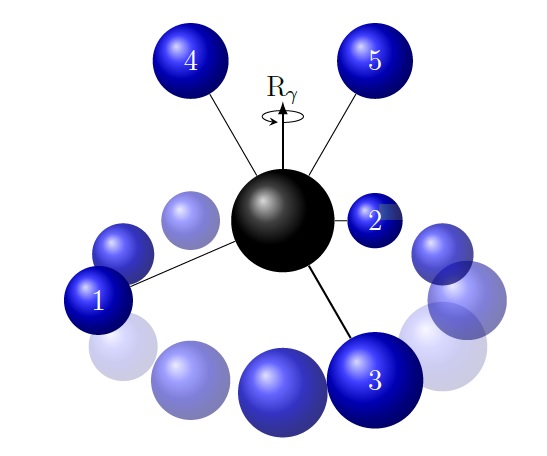
A central concept in chemistry is molecular structure. A molecule is thought of as having a well-defined structure which can be represented by the ball-and-stick models popular with chemists. In recent years, however, it has become clear that some molecules are extremely flexible: the nuclei move almost freely relative to each other and the idea of a ball-and-stick model becomes meaningless. Also, the traditional methods for the theoretical description of the rotation-vibration motion cannot be applied to an extremely flexible molecule. The best known example of an extremely flexible molecule is protonated methane CH5+. Since this ion plays an important role in the exploration of interstellar space, the understanding of its spectrum has become an important problem in theoretical spectroscopy and molecular physics. A major step forward towards this goal was taken at the University of Cologne, where Prof. Stephan Schlemmer and his group recorded high-resolution spectra of CH5+ and subjected them to an initial, statistical analysis.


Another step forward has now been made in the framework of the Ph.D. thesis of Dr. Hanno Schmiedt which was defended on January 24th, 2017. Dr. Schmiedt was jointly supervised by Prof. Schlemmer and Prof. Per Jensen at the University of Wuppertal. The new work makes a novel and very promising approach to the rotation-vibration problem in CH5+. The free rotation of the molecule in space, which represents three degrees of freedom, is treated on an equal footing with two almost-free vibrational degrees of freedom, and the resulting motion is viewed as a free rotation in five-dimensional space with the appropriate symmetry group SO(5). The mathematical theory for SO(5) rotation is known, it is already used in the theoretical description of atomic nuclei, but it is the first time that it has been applied to a molecule. With the new theory, the experimental CH5+ observations from Cologne can be satisfactorily explained and understood for the first time. The new theory has been published in the prestigious Physical Review Letters and an invited review of it has appeared as a so-called “Frontiers article” in Chemical Physics Letters.
Publications on molecular symmetry
K. L. Chubb, Per Jensen, and S. N. Yurchenko: Symmetry Adaptation of the Rotation-Vibration Theory for Linear Molecules, Symmetry 10, 137/1-23 (2018). DOI: 10.3390/sym10050137 Open access: Abstract here. Download paper here.
H. Schmiedt, Per Jensen, and S. Schlemmer: The role of angular momentum in the superrotor theory for rovibrational motion of extremely flexible molecules, J. Mol. Spectrosc. 342, 132–137 (2017). DOI: 10.1016/j.jms.2017.06.002
H. Schmiedt, Per Jensen, and S. Schlemmer: Rotation-vibration motion of extremely flexible molecules - The molecular superrotor, Chem. Phys. Lett. 672, 34–46 (2017). DOI: 10.1016/j.cplett.2017.01.045 "Frontiers article" prepared by invitation.
H. Schmiedt, Per Jensen, and S. Schlemmer: Collective molecular superrotation: A model for extremely flexible molecules applied to protonated methane, Phys. Rev. Lett., 117, 223002/1-5 (2016). DOI: 10.1103/PhysRevLett.117.223002
H. Schmiedt, S. Schlemmer, and Per Jensen: Symmetry of extremely floppy molecules: Molecular states beyond rotation-vibration separation, J. Chem. Phys. 143, 154302/1-8 (2015). DOI: 10.1063/1.4933001
H. Schmiedt, Per Jensen, and S. Schlemmer: Unifying the rotational and permutation symmetry of nuclear spin states: Schur-Weyl duality in molecular physics, J. Chem. Phys. 145, 074301/1-6 (2016). DOI: 10.1063/1.4960956
K. M. T. Yamada, Per Jensen, S. C. Ross, O. Baum, T. F. Giesen, and S. Schlemmer: The torsional and asymmetry splittings in HSOH, J. Mol. Structure 927, 96-100 (2009).
P. R. Bunker and Per Jensen: Spectroscopy and Broken Symmetry, in: "Frontiers of Molecular Spectroscopy," (J. Laane, Ed.), Elsevier, Amsterdam, 2008. Article prepared by invitation.
P. R. Bunker and Per Jensen: "Fundamentals of Molecular Symmetry," IOP Publishing, Bristol, 2004 (ISBN 0-7503-0941-5).
K. M. T. Yamada, G. Winnewisser, and Per Jensen: Internal Rotation Tunneling in HSOH, J. Mol. Structure 695-696, 323-337 (2004).
Per Jensen and P. R. Bunker: The Symmetry of Molecules, in: "Encyclopedia of Chemical Physics and Physical Chemistry" (J. H. Moore and N. D. Spencer, Eds.), IOP Publishing, Bristol, 2001. Article prepared by invitation.
Per Jensen and P. R. Bunker: Nuclear Spin Statistical Weights Revisited, Mol. Phys., 97, 821-824 (1999).
P. R. Bunker and Per Jensen: Spherical top molecules and the molecular symmetry group, Mol. Phys., 97, 255-264 (1999).
P. R. Bunker and Per Jensen: "Molecular Symmetry and Spectroscopy, 2nd Edition," NRC Research Press, Ottawa, 1998 (ISBN 0-660-17519-3).
Per Jensen and P.R. Bunker: The Molecular Symmetry Group for Molecules in High Angular Momentum States, J. Mol. Spectrosc. 164, 315 (1994).
